Especialización Farma
Farma Skills
Generics Packaging
Pharmaceutical Braille
Serialization
Multi-language
Tamper Evidence
Regulatory
Hospital products
Veterinary medicines
Generics Packaging
Pharmaceutical generics are all about:
- cost
- speed
- volume
- knowlege
Lacía Health has formed a team specialized in designing, adapting and creating print-ready files (final arts) for pharmaceutical generics with more than 5 years of experience and thousands of products timely approved.
Being Spain is one of the top countries both producing and consuming pharmaceutical generics, Lacía Health has focused on this area obtaining an excellent performance.
Pharmaceutical Braille
Braille information on primary and secondary pharmaceutical and medicinal products has long been a standard requirement. Lacía Health have been creating pharmaceutical artworks long before Braille was a standard requirement. We were also implementing pharma Braille into pharma artworks at the early stages prior to any of the now established guidelines.
Our pharma clients benefit from our years of experience knowing that we implement pharma Braille correctly and is included as part of our QA processes. We can handle Braille implementation as part of our multi-language projects and services.

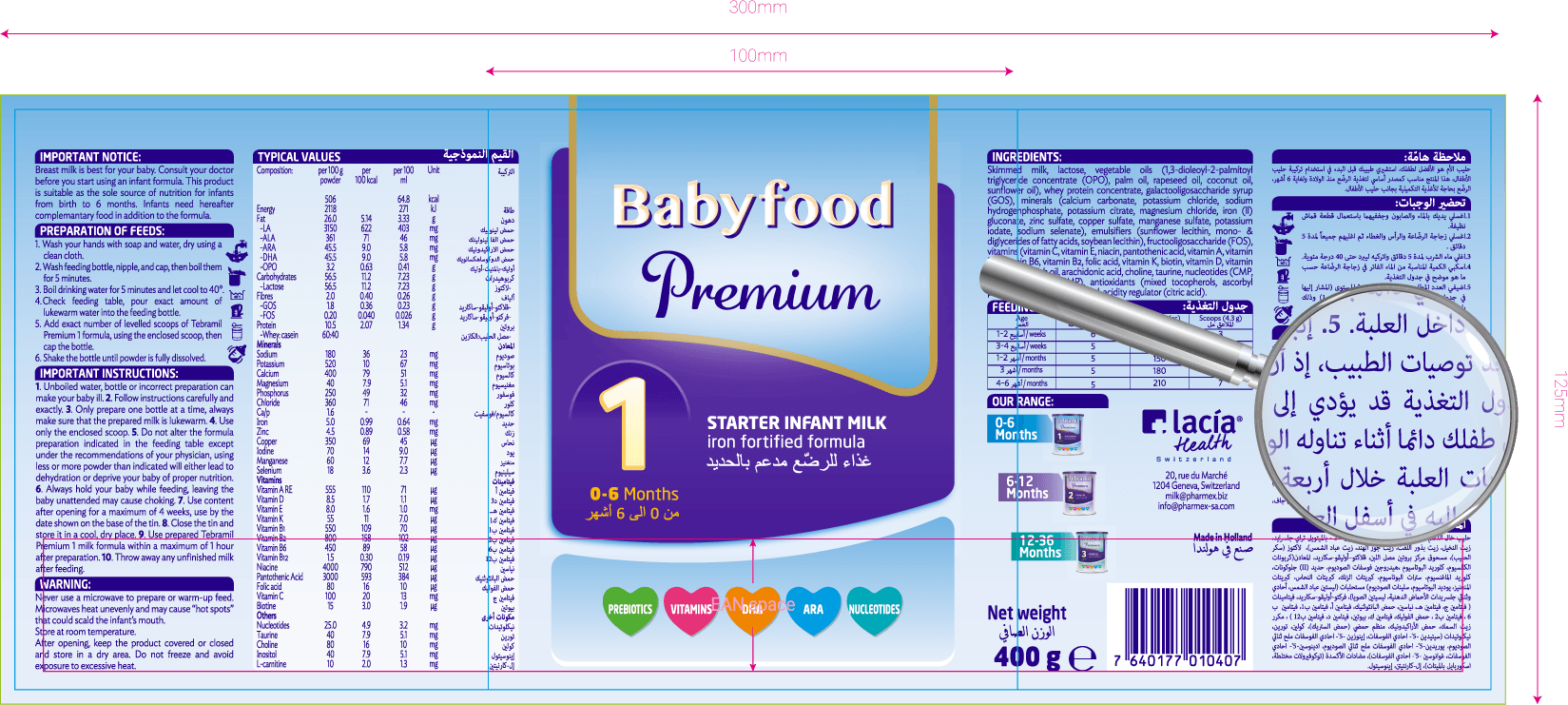
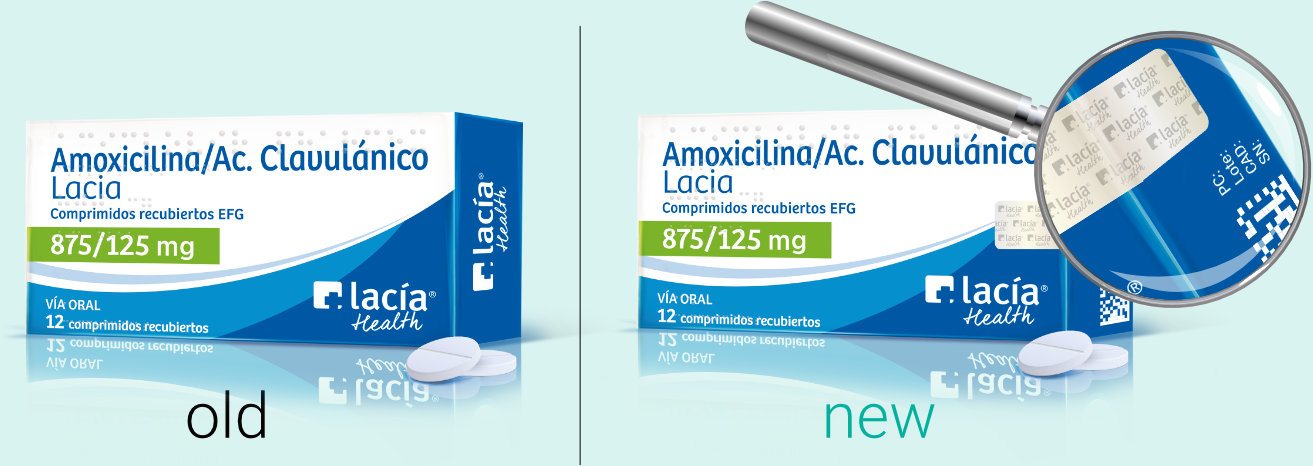
Serialization
Security devices
Serialization: we adapt artwork for later inclusion of the unique identifier within a 2D datamatrix baracode in every piece of packaging. Quite frequently these reviews are used to update packaging as well as to review designs.
Tamper Evidence
Tamper evidence: Artwork associated to every secondary pack (i.e. carton) must be reviewed according to ensure the possibility to be sealed in a tamper-evident way, which visibly exposes attempts to open them. Our team is specialized, have a thorough knowledge in pharmaceutical packaging and is able to keep up with changing regulations. They are experts in modifying artworks for timely compliance with new regulation.
Regulatory
The team at Lacía Health works directly with our clients’ REGULATORY departments to make submission and approval processes for pharmaceutical products and medical devices fast and efficient.
Differentiating design compliant with GMP
Our service supports Regulatory Affairs teams in preparing, reviewing and submitting all required artwork documentation for new products and/or changes in existing ones whilst optimizing time spent in every step of the submission and approval process.

Hospital products
As well as pharmaceutical artwork, Lacía Health helps our clients with design, artwork and creation of print-ready files for any piece of hospital products, its packaging and any associated graphical element.
Veterinary medicines
In addition to artwork & packaging design of medicines for human use, Lacía Health works in veterinary medicines projects considering GMP as it relates to labeling, artwork management and packaging design.
Multi-language
Main front and back panel carton facings, prospect, blisters…
Multi language, multiple markets, multiple branding. We’ve got it covered; whether it’s the combination of markets, varying trade names & branding, various packaging components, Lacía Health has the capacity and specialised expertise our clients need.